

The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.Ĭompeting interests: I have read the journal's policy and the authors of this manuscript have the following competing interests: Ownership in Turkey Creek Biotechnology, LLC.īacterial pathogens secrete toxins and other effectors to promote virulence by subverting host processes and defenses through the evolution of specialized secretion systems (type I to type IX). Molecular graphics and analysis of cryo-EM maps were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at UCSF, with support from NIH P41-GM103311. This work also used NE-CAT beamline 24-ID-C (GM124165) with a Pilatus detector (RR029205). Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. This research used resources of the Advanced Photon Source, a U.S. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.ĭata Availability: Crystallographic structures and data are available with accession numbers 6WA6 and 6WA9 from the RCSB/pdb ( Funding: The work was supported by awards 1R01 AI108778 and R01 EY022052 to BWS and AR ( JLJ was supported by the Training in Gastroenterology Training Grant, DK007673. Received: ApAccepted: AugPublished: October 13, 2020Ĭopyright: © 2020 Jensen et al. PLoS Pathog 16(10):Įditor: Zhao-Qing Luo, Purdue University, UNITED STATES
These results provide novel insights into the molecular mechanisms governing active substrate recognition and translocation through a T3SS.Ĭitation: Jensen JL, Yamini S, Rietsch A, Spiller BW (2020) “The structure of the Type III secretion system export gate with CdsO, an ATPase lever arm”. We also demonstrate through structure-based mutagenesis of the homologous export gate in Pseudomonas aeruginosa that mutation of this interface disrupts effector secretion. This structure defines the interface between these essential T3S proteins and reveals that CdsO engages the periphery of the export gate that may allow the ATPase to catalyze an opening between export gate subunits to allow cargo to enter the export apparatus. We present the structure of the soluble export gate homo-nonamer, CdsV, in complex with the central stalk protein, CdsO, of its cognate ATPase, both derived from Chlamydia pneumoniae.
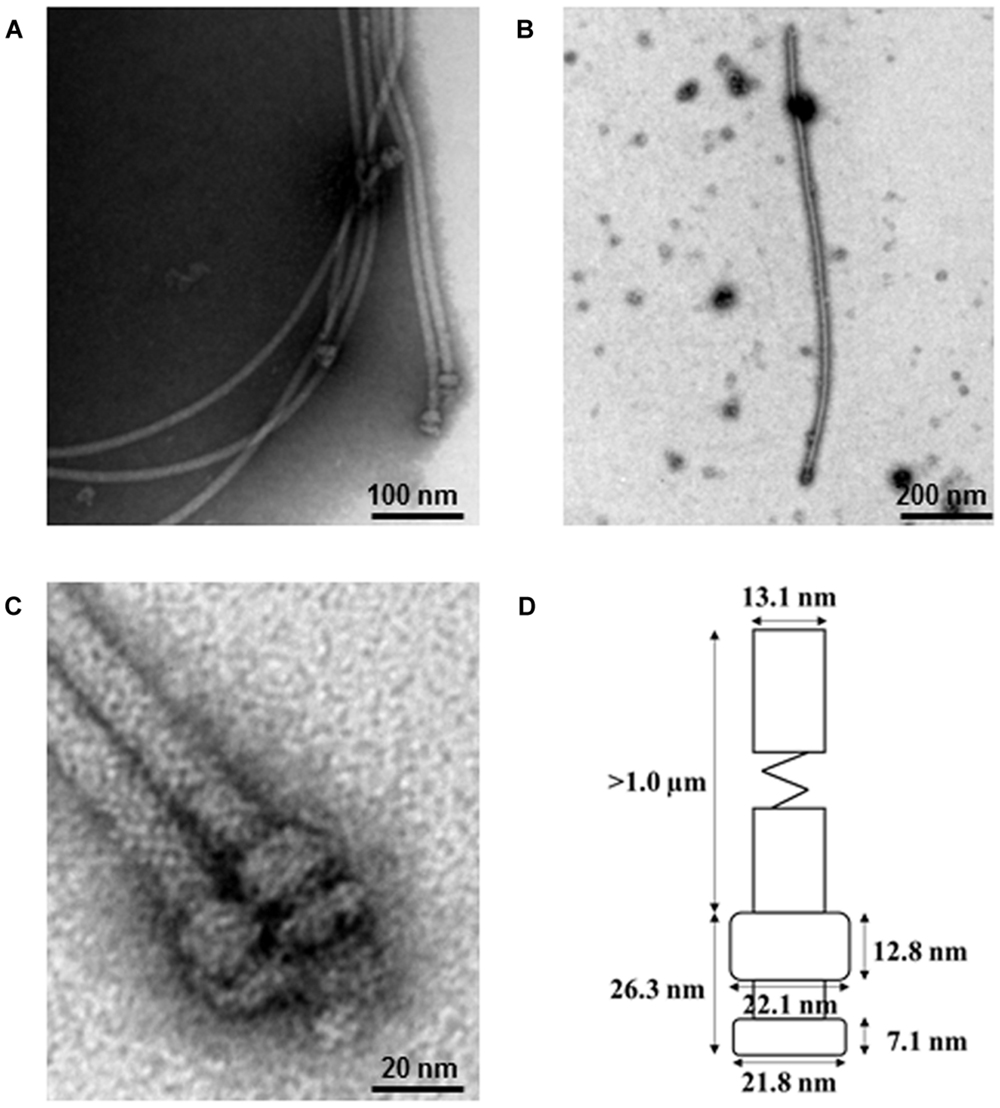
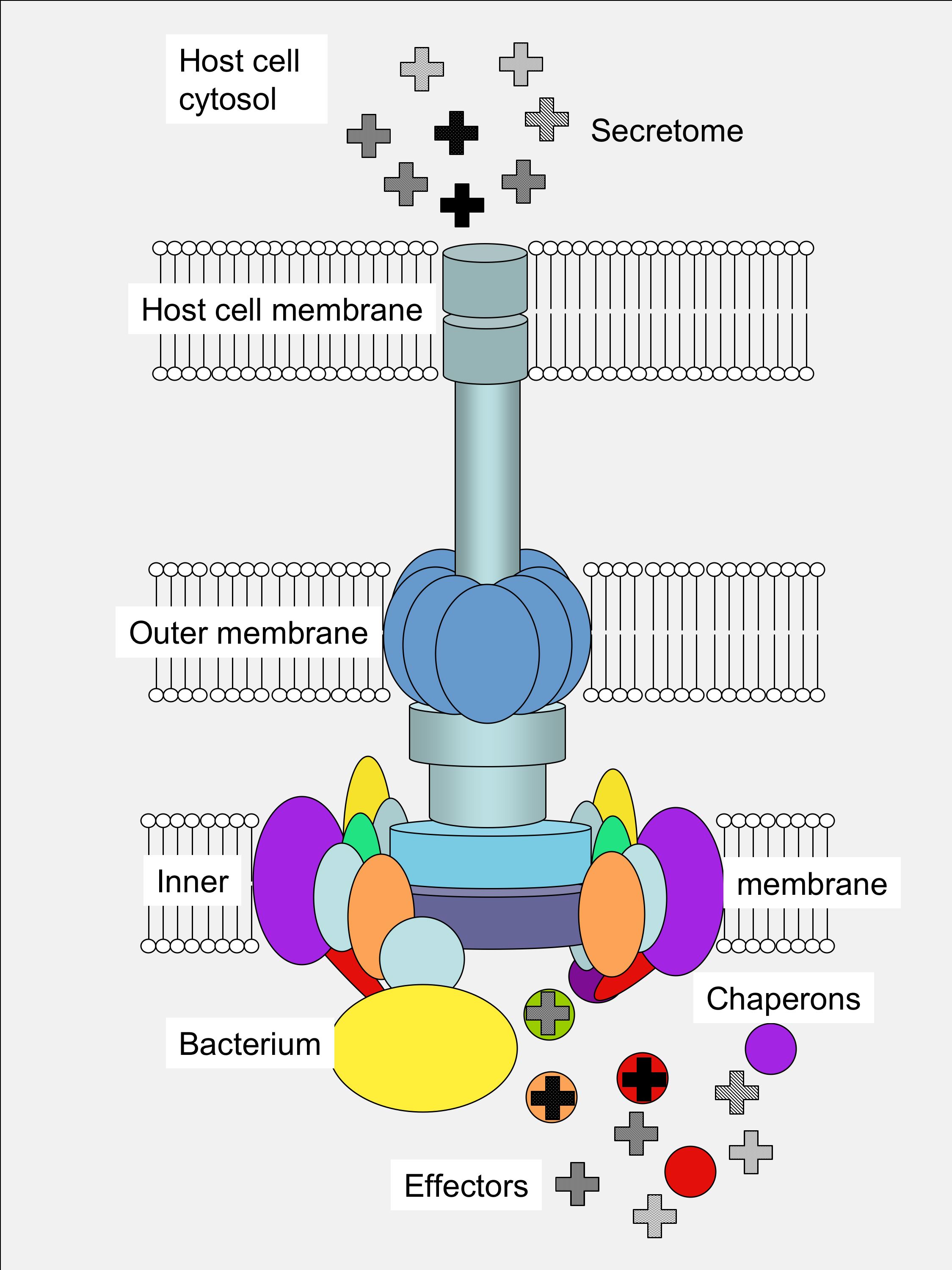
Two components critical for efficient effector export are the export gate protein and the ATPase, which are proposed to be linked by the central stalk protein of the ATPase. Cytosolic components of T3SS include a portion of the export apparatus, which traverses the inner membrane and features the opening of the secretion channel, and the sorting complex for substrate recognition and for providing the energetics required for protein secretion. Type III protein secretion systems (T3SS) deliver effector proteins from the Gram-negative bacterial cytoplasm into a eukaryotic host cell through a syringe-like, multi-protein nanomachine.


 0 kommentar(er)
0 kommentar(er)
